Aspergillosis in Adult
Alerts and Notices
Important News & Links
Synopsis

Aspergillus, a hyaline mold, is found ubiquitously worldwide in decaying vegetation, soil, water, food, and plants. Inhalation of Aspergillus conidia into the lungs is the most common mode of acquisition. The varied pulmonary manifestations of infection depend to a large degree on the immune status of the host. They range from colonization leading to tracheobronchitis or a fungus ball to invasive disease in immunocompromised patients. There also can be an allergic response as with allergic bronchopulmonary aspergillosis (ABPA).
Aspergillus tracheobronchitis, which occurs in lung transplant recipients, can be asymptomatic but may cause fever, cough, wheezing, and hemoptysis.
An aspergilloma, sometimes referred to as chronic pulmonary aspergillosis or mycetoma (fungus ball), is a solid clump of mold growing in a preexisting pulmonary cavity. This is most common in an immunocompetent host, developing more frequently in persons with structural lung disease. It presents clinically with cough and hemoptysis.
ABPA syndrome is characterized by transient pulmonary infiltrates and by exacerbation of dyspnea, wheezing, and cough in chronic asthmatics and occasionally in patients with cystic fibrosis.
Patients with prolonged and profound neutropenia (absolute neutrophil count < 100/mm3), those undergoing hematopoietic stem cell transplantation (HSCT), particularly those with graft-versus-host disease (GVHD), patients on chronic high dose corticosteroids, patients with chronic granulomatous disease, solid organ transplant recipients (especially lung), and those on newer immunosuppressives such as tumor necrosis factor (TNF)-alpha antagonists or T-cell ablative agents (alemtuzumab, antithymocyte globulins) are at risk for invasive disease. Recently, COVID-associated pulmonary aspergillosis has been described, but it is unclear if this is related to the SARS-CoV-2 virus or the steroid regimen that patients with severe COVID-19 pneumonia receive for management.
Invasive aspergillosis most commonly begins in the airway (sinuses or lungs), and dissemination can occur by hematogenous route or as a result of local extension. Dissemination can lead to central nervous system, liver, spleen, heart, bone, and skin involvement. Invasive pulmonary aspergillosis often presents with asymptomatic fever and incidental radiographic findings but can also present with dry cough, dyspnea, pleuritic chest pain, hemoptysis, or fever. These symptoms can resemble those of pulmonary embolism, reflecting the angioinvasive nature of the disease.
A locally invasive or semi-invasive form of the disease that lacks vascular invasion or distant dissemination is also recognized. This is an indolent process that develops over months or years, hence its other name, chronic necrotizing aspergillosis.
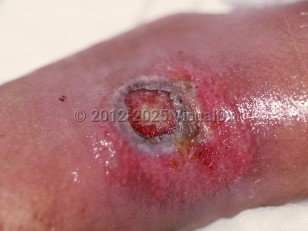
Cutaneous aspergillosis begins as red papules that form pustules. These pustules ulcerate and leave a central eschar evocative of the diagnosis.
Codes
B44.9 – Aspergillosis, unspecified
SNOMEDCT:
65553006 – Aspergillosis
Look For
Subscription Required
Diagnostic Pearls
Subscription Required
Differential Diagnosis & Pitfalls

Subscription Required
Best Tests
Subscription Required
Management Pearls
Subscription Required
Therapy
Subscription Required
Drug Reaction Data
Subscription Required
References
Subscription Required
Last Updated:11/16/2021